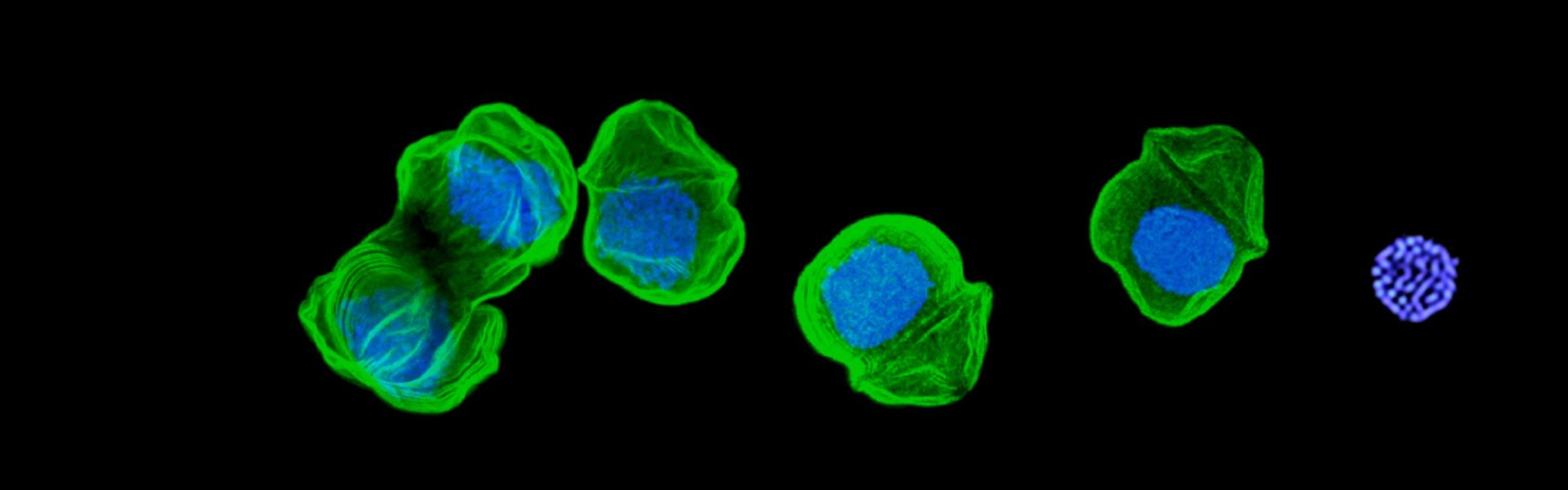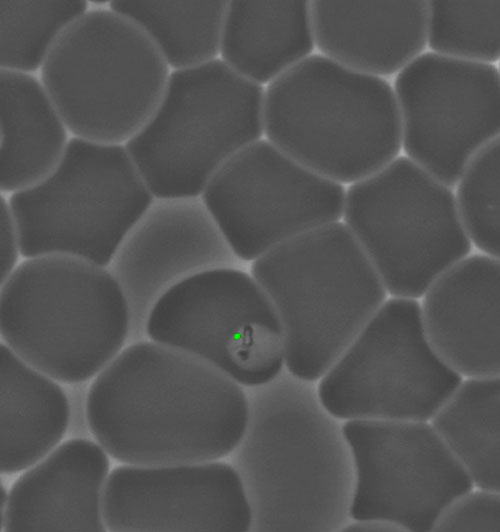
A non-photosynthetic plastid in the malaria parasite (Plasmodium) in its blood cell host.
Selected publications:
- Waller, R.F. and Kořený, L. (2017) Plastid Complexity in Dinoflagellates: A Picture of Gains, Losses, Replacements and Revisions. In Secondary Endosymbioses (ed Yoshihisa Hirakawa), Vol 84, Advances in Botanical Research, UK: Academic Press, 2017, pp. 105-144
- Waller, R.F. et al (2016) Metabolic pathway redundancy within the apicomplexan-dinoflagellate radiation argues against an ancient chromalveolate plastid. Comm. Integrat. Biol. 9: e1116653
- Gornik, S.G. et al (2015) Endosymbiosis undone by stepwise elimination of the plastid in a parasitic dinoflagellate. PNAS 112: 5767-72
- Hewitt, V et al (2014) Modifications and innovations in the evolution of mitochondrial protein import pathways. In Endosymbiosis (ed Wolfgang Loffelhardt) Springer, New York, USA pp 19-25
- Danne, J.C. et al (2013) Alveolate mitochondrial metabolic evolution: dinoflagellates force reassessment of the role of parasitism as a driver of change in apicomplexans. Mol. Biol. Evol. 30(1): 123-39
- Danne, J.C. et al (2011) Analysis of dinoflagellate mitochondrial protein sorting signals indicates a highly stable protein targeting system across eukaryotic diversity. J. Mol. Biol. 408: 643-53
- Waller, R.F. et al (2009) Evidence of a reduced and modified mitochondrial protein import apparatus in microsporidian mitosomes. Euk. Cell 8: 19-26
- Gould, S.B. (2008) Plastid Evolution. Ann. Rev. Plant Biol. 59:491-517
- Patron, N.J. et al (2007) Transit peptide diversity and divergence: a global analysis of plastid targeting signals. BioEssays 29:1048-58
- Patron, N.J., et al (2006) A tertiary plastid uses genes from two endosymbionts. J. Mol. Biol. 357:1373-82
- Patron, N.J. et al (2005) Complex protein targeting to dinoflagellate plastids. J. Mol. Biol. 348:1015-24
- Waller, R.F. et al (2005) The apicoplast: a review of the derived plastid of apicomplexan parasites. Curr. Issues Mol. Biol. 7: 57-79
- Ralph, S.R. et al (2004) Metabolic maps and functions of the Plasmodium falciparum apicoplast. Nature Rev. Micro. 2: 203-16
- Gentle, I. et al. (2004) The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J. Cell Biol. 164:19-24
- Waller, R.F. et al (2003) A green algal apicoplast ancestor. Science 301: 49a-50a
- Waller, R.F. et al (2003) A type II pathway for fatty acid biosynthesis presents drug targets in Plasmodium falciparum. Antimicrob. Agents Chemoth. 47: 297-301
- Waller, R.F. et al (2000) Protein trafficking to the plastid of Plasmodium falciparum is via the secretory pathway. EMBO J. 19: 1794-1802
- Keeling, P.J. et al (1999) Shikimate in apicomplexan parasites. Nature 397: 219-220
- Waller, R.F. et al. (1998) Nuclear-encoded proteins target to the plastid in Toxoplamsa gondii and Plasmodium falciparum. Proc. Natl Acad. Sci. USA 95: 12352-57
Organelle gain and evolution
All modern eukaryotic cells represent mergers of at least two formerly independent cells, one within the other. The internalised cells become endosymbionts, and endosymbiotic bacteria have become mitochondria and plastids. Eukaryotes and their organelles can themselves become endosymbionts in a serial process that resembles cellular Russian matryoshka dolls. How many times this has happened in eukaryotic evolution is still unknown.
Each of these cellular acquisitions provides novel genetic and metabolic capabilities to the host cell, and these events have been major driving forces in the success and diversification of modern eukaryotes. But forming stable cellular partnerships is a highly complicated affair, with disparate genetic, metabolic and cellular processes needing to be brought into alignment within the one cell. The Waller lab has a long history of studying these evolutionary processes and their implications, including:
- gene relocation from endosymbiont to host nucleus
- protein trafficking back into the endosymbiotic organelles
- the balance of metabolic processes between host cell and symbiont
- adaptation of chloroplasts and mitochondria in parasite lineages
- organelle reduction and even loss
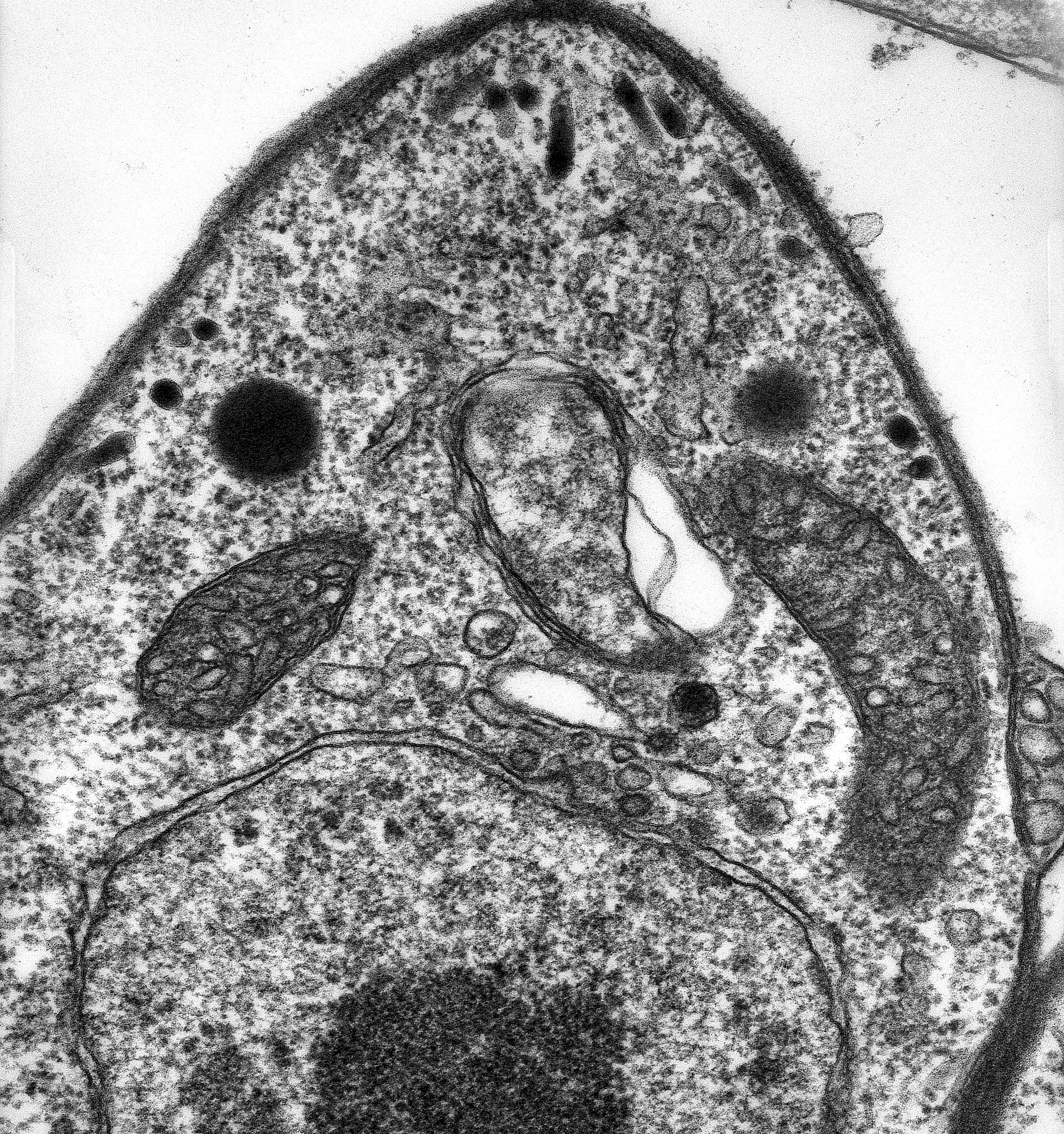
Toxoplasma gondii thin section showing its plastid flanked by two mitochondrial profiles