CELL COMPARTMENTALISATION AS A PLATFORM FOR INNOVATION
Cell compartmentalisation is the foundation of eukaryotic evolution and innovation. It has been achieved by multiple mechanisms, including elaboration of invaginated membranes forming discrete but dynamic intracellular spaces, and formation of cytoskeletal structures that define cell domains, scaffolds and sites of specialised activities. This compartmentalisation enables segregation of cell functions that might otherwise be impractical or incompatible and, thus, tremendous development of complexity of function within these different cell spaces and diversity of eukaryotic organisms.
Apicomplexan protistan parasites have displayed superb ingenuity for generation and specialisation of cell compartments. Apicomplexa comprises a phylum of over 6000 obligate intracellular parasites that cause devastating disease in humans, including malaria, toxoplasmosis and cryptosporidiosis, and significant impact on food security as animal pathogens. Novel and adapted cell structures and compartments are key to the success of apicomplexans as pathogens driving processes such as host cell invasion, defence against host immunity, and rapid parasite proliferation. Further tuning of these compartments has allowed a very wide range of host organisms amongst apicomplexans.
To better understand, manage and combat the diseases caused by apicomplexans, the Waller lab is using a number of sub-cellular and whole cell spatial proteomics methods to capture the complexity of apicomplexan cell structures and compartments. We are then applying powerful genetic tools to investigate the molecular mechanisms that underpin the functions that these organisations provide. Comparative studies, both within apicomplexans but also with the related dinoflagellates, provides powerful insight into the evolution of these eukaryotes including ancestral, relatively stable compartments and those that are evolving and adapting quickly.
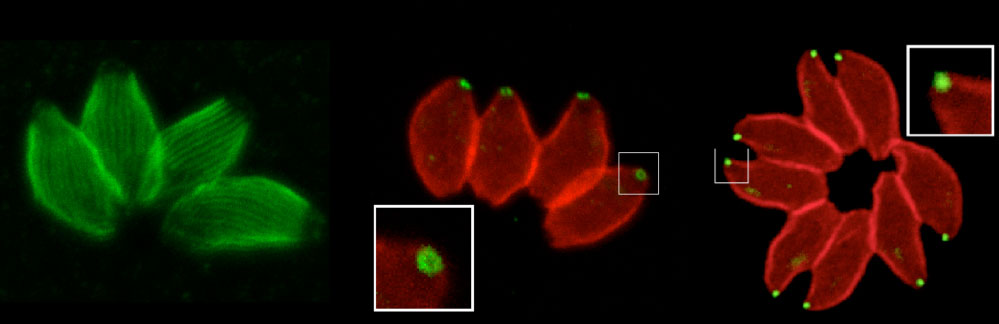
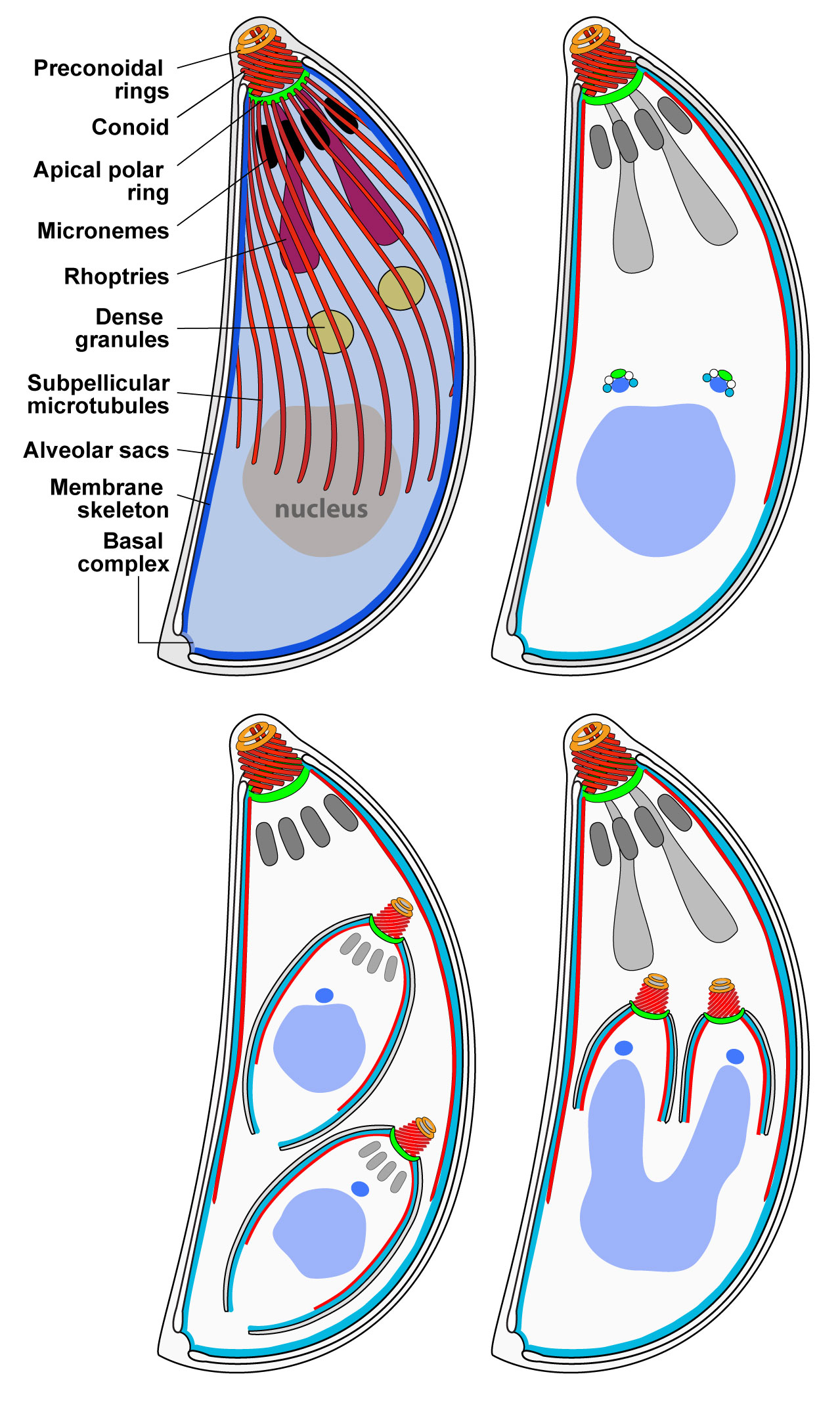
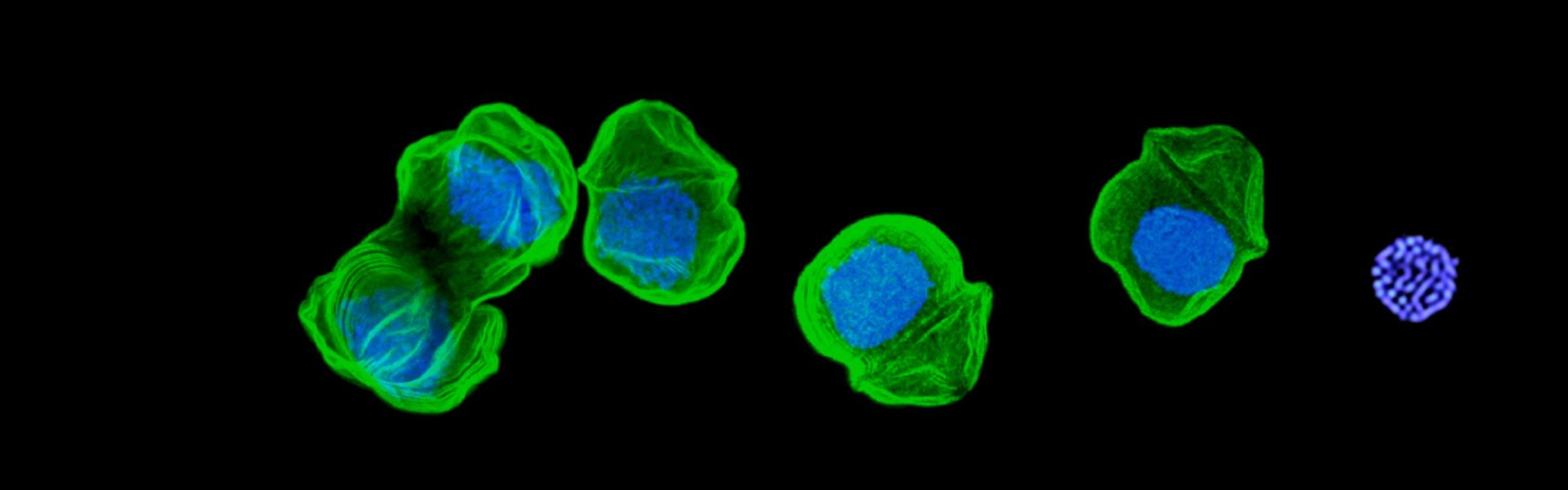
The organisation of Toxoplasma compartments and cellular structures are central to both host cell invasion and parasite cell replication
Selected publications:
- Wall, R.J et al (2016) SAS6-like protein in Plasmodium indicates that conoid-associated apical complex proteins persist in invasive stages within the mosquito vector. Sci. Rep. 6:28604
- Katris, N.J. et al (2014) The apical complex provides a regulated gateway for secretion of invasion factors in Toxoplasma. PLOS Pathog. 10: e1004074
- El-Haddad, et al (2013) Characterization of TtALV2, an essential charged repeat motif protein of the Tetrahymena thermophila membrane skeleton. Euk. Cell 12(6): 932-40
- Gould. S.B. et al (2011) Ciliate pellicular proteome identifies novel protein families with characteristic repeat motIfs that are common to Alveolates. Mol. Biol. Evol. 28: 1319-31
- Gould, S.B., et al (2008) Alveolins, a New Family of Cortical Proteins that Define the Protist Infrakingdom Alveolata. Mol. Biol. Evol. 25:1219-30